In Brief
Clinical trials bridge the gap between a concept in a lab and lifesaving drugs and therapeutics being available to the masses. Without them, medical advances cannot be made. Yet, participating in a clinical trial is not always easy or enjoyable for patients. They often undergo more frequent or longer treatment visits and risk insurance claims denials due to improper coding. As a result, recruitment and retention of patients is a significant challenge.
By creating a patient-centric clinical trial experience that reduces the barriers facing participants, research institutions can increase participation and ultimately bring medical advances to the public sooner. Huron surveyed over 25 leading research institutions to gain a better understanding of their current and planned practices for improving the participant experience. The survey discussed the four main stages of engagement for clinical trials participants:
- Awareness
- Recruitment
- Retention
- Follow Up
It’s not enough to make incremental improvements to the participant experience, instead institutions must fundamentally shift the way they design the experience. They must begin to view participants as consumers and design consumer-centric research models that make all aspects of clinical trial participation easy and convenient. Creating a better experience for participants will also make it is easier for investigators to recruit into studies.
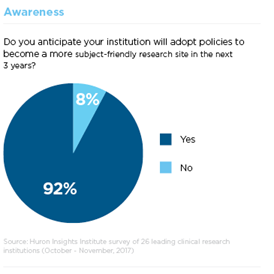
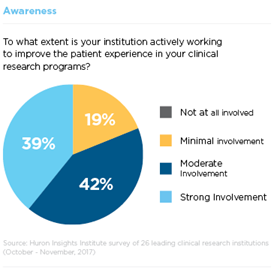
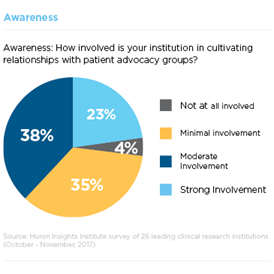
Step 1: Awareness
Participant enrollment remains one of the most significant challenges for clinical research sites. Of the trials registered in ClinicalTrials.gov that were closed or terminated in 2011, 19 percent failed to meet accrual goals (e.g., 85 percent of expected enrollment) or were terminated early due to insufficient accrual. This is not surprising considering that 50 percent of Americans are not aware of clinical trials.
To address this problem, many institutions Huron surveyed are taking steps to improve their recruitment rates. 60 percent reported moderate to strong involvement in cultivating relationships with participant advocacy groups. Leaders are also planning to improve community engagement and conduct more locally relevant research.
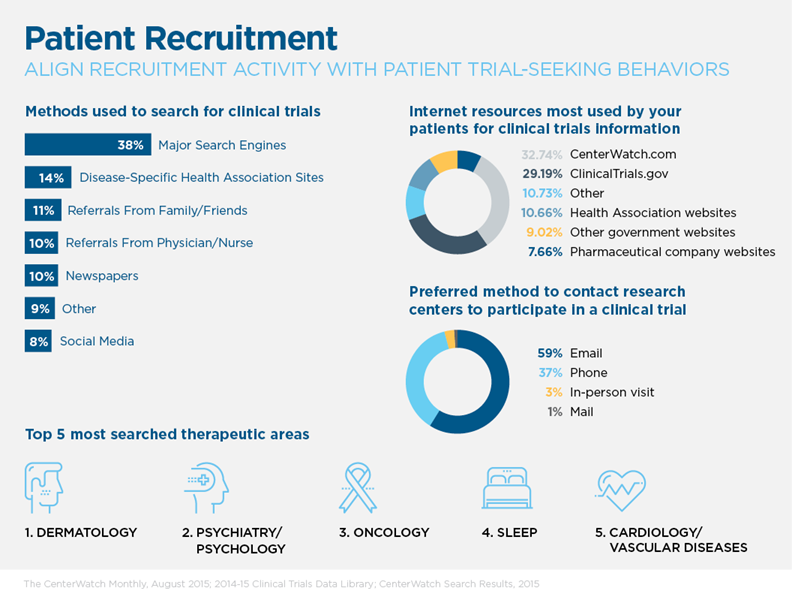
Stage 2: Recruitment
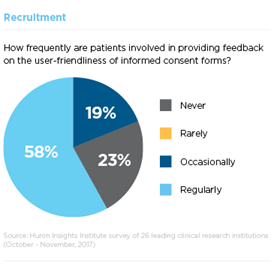
Consumers want their buying experience to be easy, efficient and pleasant. Clinical trials enrollment is often anything but. Participants are more likely to become engaged in research if research sites write recruitment materials in lay person’s terms, utilize images that reflect participant diversity, clearly explain how research is used and solicit participant feedback on forms and processes. Yet fewer than one in five research sites surveyed obtain regular participant feedback on informed consent documents and many researchers struggle with how to approach participants.
UMHealthResearch is a University of Michigan website that makes clinical trial enrollment feel more like purchasing a product than enrolling in a clinical trial. The website uses simple language to demystify clinical trials and clinical trial descriptions are written in lay language. Individuals can browse clinical trials, ask questions and request to enroll. They can also share their health data via a secure portal and receive an email listing clinical trials that they may qualify for. By thinking about the participant experience like retailers think about the consumer experience, UMHealthResearch has made enrollment simple which is also making it easier for investigators to recruit participants.
Stage 3: Retention
Once institutions recruit participants into trials, they must contend with the risk of participants dropping out. Clinical trials have a dropout rate of 30 percent. Retention hinges on participants having a positive, convenient study experience. Research sites often overlook this critical aspect of the participant experience. Less than five percent have services to help participants manage trial commitments with their jobs and less than one third of respondents provide special services to support the families of participants (e.g., lounges, meals, accommodations for overnight stays, etc.). As a result, the level of participant satisfaction attributed to clinical trials is comparable to industries with some of the worst customer satisfaction rates including internet service providers, utility companies and health insurers.
By focusing on the participant experience rather than just the trial itself, sites can keep participants engaged throughout the research process. Ikea sells home goods, but it also sells a customer experience. Their stores have similar layouts that were designed with the consumer in mind. They offer supervised children’s play areas, food courts, an app to help customers navigate the store, and an engaging layout that encourages consumers to browse and delivery services. Like Ikea, institutions should think about how to eliminate barriers to participation by making participation as simple as possible.
Tapping into technology can also simplify clinical trial participation. Apple and Stanford Medicine are recruiting participants into a trial to see if the Apple Watch can detect atrial fibrillation. Participants simply wear the watch and their data is collected. For participants, this is a better experience that offers minimal disruption to their daily routines.
Stage 4: Referrals
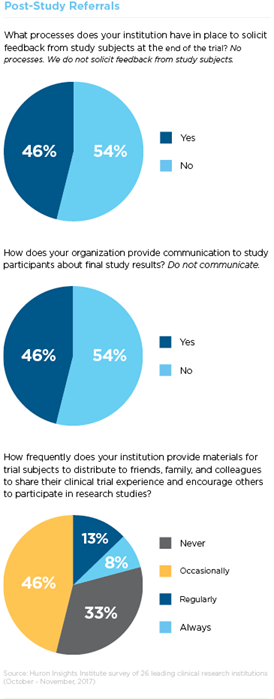
In one study, 97 percent of clinical trial participants said they would definitely or probably recommend a clinical trial to a friend or relative. However, only 20 percent of the institutions surveyed by Huron regularly or always provide materials for trial participants to distribute to friends, family and colleagues and just 46 percent of institutions communicate to study participants about final study results.
While those who participate are happy they did so, only 40 percent of Americans have a positive impression of clinical trials. By continuing to engage with participants, institutions can begin to create life-long relationships with these consumers and build a positive image within the community. Much like retailers have tapped into bloggers as brand ambassadors to establish credibility of their brand, clinical trials participants can be the same for institutions. These individuals can help bring in new participants by sharing their stories while continuing to participate in clinical trials.
Put the Patient First
Recruitment and retention remain significant challenges for research institutions that must be addressed. By treating participants like consumers and putting them at the center of all aspects of research, institutions can overcome these obstacles and transform the clinical trial experience to one that is simple and user friendly, speeding up the rate at which lifesaving cures are made.
KEY TAKEAWAYS
-
Think differently.
Shift your mindset around those participating in clinical trials from one of research subjects to consumers. -
Plan differently.
Consider ways to create a more consumer-centric experience through all stages of clinical trials. -
Act differently.
Invest in services and implement procedural changes to transform the research experience.